crwdns2944766:0crwdne2944766:0
crwdns2918718:0crwdne2918718:0crwdns2935091:0crwdnd2935091:0crwdne2935091:0
Background
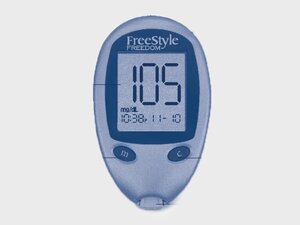
Abbott Freestyle Freedom is a blood glucose meter used to measure blood sugar levels at home. Its large display makes it easy to read. Its portable and compact size is designed to fit comfortably in your hands. It has a Mode and Configure button just below the display and the test strip port is at the bottom of the device.
The FreeStyle Freedom Meter should only be used with FreeStyle Test Strips and FreeStyle Control Solution. Using other brands could result in inaccurate measurements.
The device can be used for measuring blood sugar levels from blood samples taken from the forearm, upper arm, hand, thigh, calf, or fingers.
This model has been discontinued and is no longer in production.
An alternative model - the Abbott Freestyle Freedom Lite - is available in outlets like CVS for $22.99
Identification
The Abbott Freestyle Freedom model can be identified by the model name "Freestyle Freedom" above the display screen.
Technical Specification
Automatic Shutoff: Two minutes after last user action
Battery Life: 1,000 tests
Measurement Units: mg/dL (milligrams per deciliter)
Meter Storage Temp: -4º to 140º F (-20º to 60º C)
Memory: 250 blood glucose and control solution tests with date and time
Operating Temperature: 40º to 104º F (5º to 40º C)
Power Source: One #2032 battery (3 volt lithium replaceable)
Result Range: 20 to 500 mg/dL
Sample Size: 0.3 microliter (300 nanoliters)
Size: Width 2.00” x Length 3.30” x Thickness 0.63” (at thickest point)
Test Time: Average of 5 seconds
Weight: 1.43 oz. (including battery)